Genotypic Frequency of Rh Cw Antigen in Blood Donors of Northern Pakistan
By Sarah Fatimah1, Asad Mahmood Abbasi1, Ali Abbasi2, Nargis Sabir3, Rozina Ghani4, Rehan Ahmed Khan Lodhi1Affiliations
doi: 10.29271/jcpsp.2024.04.419ABSTRACT
Objective: To determine the genotypic frequency of Rh Cw antigen in blood donors of Northern Pakistan.
Study Design: Descriptive cross-sectional study.
Place and Duration of the Study: Department of Molecular Haematology, Armed Forces Institute of Transfusion (AFIT), Rawalpindi, Pakistan, from August 2022 to January 2023.
Methodology: Blood donors were randomly selected. Venous blood samples were taken in K3-EDTA anticoagulant tubes. ABO and Rh D grouping were performed conventionally. DNA for Rh Cw genotyping was extracted via Chelex TM, followed by PCR amplification using an ABI 2700 thermal cycler. Human growth hormone (HGH) acts as an internal control. Amplified products underwent Polyacrylamide gel Electrophoresis (PAGE).
Results: There were 400 randomly chosen donors whose ages ranged from 26-35 years, with a predominantly male population (94.8%) of Punjabi origin (67.8%). The majority (87.3%) was RhD positive. Blood group B was the most prevalent (35%) in the studied population, followed by O (34.75%). Only 1.5% had Rh Cw antigen. Rh Cw was more prevalent in ABO-positive participants (87.25%) compared to ABO-negative (12.75%).
Conclusion: There was a 1.5% prevalence of Rh Cw antigen genotype in randomly selected Northern Pakistani blood donors. Rh Cw prevalence was higher in ABO-positive participants. Significant correlation (<0.05) existed between RhD and Cw antigens. Given the implications of anti-Cw antibody, including Cw antigen-positive cells in antibody screening is recommended.
Key Words: Alloimmunisation, Blood donors, HDFN, Phenotype, Rh antigens, Transfusion.
INTRODUCTION
With about 61 antigens, the Rh blood group system stands as the largest among the 43 blood group systems recognised by the ISBT working group.1,2
Rh blood group antigens, notably Rh D, C, c, E, and e, are vital for blood transfusion compatibility and maternal-fetal interactions during pregnancy. Unlike ABO system antibodies, Rh antibodies (IgG type) arise after exposure to the foreign RBCs, significantly contributing to the Haemolytic Disease of the Newborn (HDFN) risk due to Rh antigen immunogenicity.3
These Rh blood group antigens are carried by non-glycosylated hydrophobic transmembrane proteins, specifically RhD and RhCE. These proteins, composed of 416 amino acids, traverse the cell membrane a total of 12 times. The striking similarity between Rh D and Rh CE is marked by a mere 32 to 35 amino acid differences.4 While the Rh D protein bears the D antigen, rich in at least 30 epitopes, the Rh CE protein is associated with CE antigens that manifest in various combinations, namely ce, Ce, cE, and CE. The crucial epitopes responsible for the C and c antigens are situated on the second extracellular loop of the Rh CE protein. On the other hand, the E and e antigens find their epitopes on the fourth extracellular loop. These integral transmembrane proteins confer stability to the cell membrane while playing an essential role in antigenic specificity.5
The absence or presence of the RHD gene is responsible for the Rh D-positive/negative polymorphism.6,7 Polymorphic antigens, such as C/c and E/e antigens, arise due to various nucleotide substitutions in the RHCE gene. The exact positioning of these polymorphisms impacts the formation of specific antigens, giving rise to a diverse landscape of antigenic profiles.
The Cw antigen, also denoted as RH:8 in ISBT nomenclature, has been of particular interest since its first documentation in 1946. This antigen's expression is linked to point mutations in the RHCE gene. The prevalence of Cw antigen varies across populations, with a higher frequency observed in Latvians and Fins (5-7%) compared to the white population (2%). Anti-Cw antibodies, albeit rare, have shown clinical significance in causing haemolytic disease of the fetus and newborn, posing potential complications during pregnancies.
Genotypic assays, targeting single nucleotide polymorphisms (SNPs), facilitate comprehensive blood profiling; particularly useful for rare antigens.8 The distribution of blood group antigens, predominantly determined by SNPs, has streamlined the design and interpretation of genotypic assays. Commercial DNA arrays have been developed to target specific antigens, enabling a comprehensive analysis of blood samples for phenotypic and genotypic studies.9,10 The emergence of these genotyping tools has paved the way for a more precise understanding of antigenic profiles.
Uptill now, no investigation has been conducted regarding the frequency of the Cw antigen in the context of Northern Pakistan. The study aimed to explore Rh Cw antigen frequency in Northern Pakistan.
METHODOLOGY
A cross-sectional study was carried out at the Molecular Haematology Department, Armed Forces Institute of Transfusion, (AFIT) with approval from the ethical review committee from August 2022 to January 2023. The confidentiality of data was ensured. Using the World Health Organisation (WHO) sample size calculator with a population of 50,000 and a confidence level of 95%, the sample size was determined. The study used a non-probability consecutive sampling technique. A detailed questionnaire was developed to record all the demographic variables (age, ethnicity, and residence etc.). Relevant history was taken from all the participants and incorporated in the study conferring to the designated questionnaire.
All blood donors (both male and female) aged 18-65 years who were willing to participate and were found fit for blood donation after fulfilling the criteria of uniform donor questionnaires, general physical examination, complete blood count (CBC), and infectious disease screening were included in the study. All donors who had been deferred on uniform donor questionnaires, verbal screening and general physical examination, and donors of single donor platelets (SDP) were excluded from the study.
A 5 ml whole blood sample was collected from ante-cubital vein. Out of it, 2 ml was transferred immediately to a tube containing EDTA and 3 ml was collected in plain tube. All blood donors were subjected to blood grouping, which included the ABO and Rh blood group determination. The ABO and Rh D blood grouping was performed on EDTA samples using serological techniques manually and then confirmed by semi-automated analyser, QasarIV®.
The ChelexTM Method was employed for DNA extraction, involving several steps.11 Initially, three millilitres of EDTA sample were centrifuged at 5000 RPM for a minute to extract white blood cells (WBCs). These WBCs (300 microliters) were obtained from the buffy coat using a micropipette and placed in a 1.5-milliliter Eppendorf tube. To lyse red blood cells (RBCs), 900 microliters of distilled water were added, followed by centrifugation at 5000 RPM to pellet the WBCs. The red cell lysis step was repeated thrice, with 300 microliters of 5% ChelexTM solution added to the white cell pellet afterwards. The tube was vortexed and heated at 95°C for 20 minutes, followed by centrifugation at 10000 RPM for two minutes. The supernatant containing DNA was stored at -70°C for amplification.
The Polymerase Chain Reaction with Allele-Specific Primers (PCR-ASP) method involves amplifying a specific DNA region. This entailed denaturation at 94°C, where double-stranded DNA templates separated, followed by annealing at 60-65°C, during which DNA primers bound to target flanking regions. Subsequently, extension occurred at 72°C, facilitated by DNA polymerase elongating primer ends along template strands. These steps were cycled 25-35 times to exponentially amplify target DNA. A "negative control" was included to detect contamination, and an internal human growth hormone (HGH) control ensured PCR efficiency.
Preventing cross-contamination, PCR, DNA extraction, and gel electrophoresis were conducted separately. For gel electrophoresis, cleaned glass plates were assembled, and a solution of 6% Polyacrylamide, Ammonium persulfate, and TEMED was poured between plates to create wells. After polymerisation, samples were loaded with bromophenol dye. Gel electrophoresis was carried out at 200 volts for 30 minutes. Following electrophoresis, gel plates were treated with Silver nitrate and a developing solution to visualise DNA bands. The results were documented after drying the gel.
Statistical Packages for Social Sciences (SPSS) version 26.0 was used for analysis of data. Percentages were calculated for qualitative data while mean and standard deviation were calculated for quantitative data. The data were presented in the form of graphs and tables. The Chi-square test was used to evaluate the differences in frequencies. A p <0.05 was considered significant. McNemar test was used to find the correlation between blood group phenotypes and the genotypic expression of Rh Cw antigen.
RESULTS
A number of 400 blood donors were enrolled in this study to determine the genotypic frequency of Cw antigens during the study period. There were 379 (94.8%) males and 21 (5.2%) females. The result of this study showed that most of the participants were males aged between 26-35 years (40.5%). The ethnic distribution revealed Punjabis to be the most common donors (67.8%), followed by Pashtuns (10.7%). Additionally, 45% of the study population was urban as shown in Table I.
Figure 1 describes that out of 400 participants, 140 (35%) had B blood group, 139 (34.8) had O blood group, and 85 (21.3%) had A blood group. However, a few participants 36 (9%) had AB blood group. It was found that 349 (87.3%) participants were RhD Positive while only 51 (12%) were Rh D negative.
Genotypic analysis showed that only 6 (1.5%) out of the 400 study participants had Rh Cw antigen while 394 (98.5%) did not have Cw antigen. There was a high prevalence of Rh Cw antigen in ABO-positive study participants as compared to the ABO-negative (p <0.05) as depicted in Figure 1.
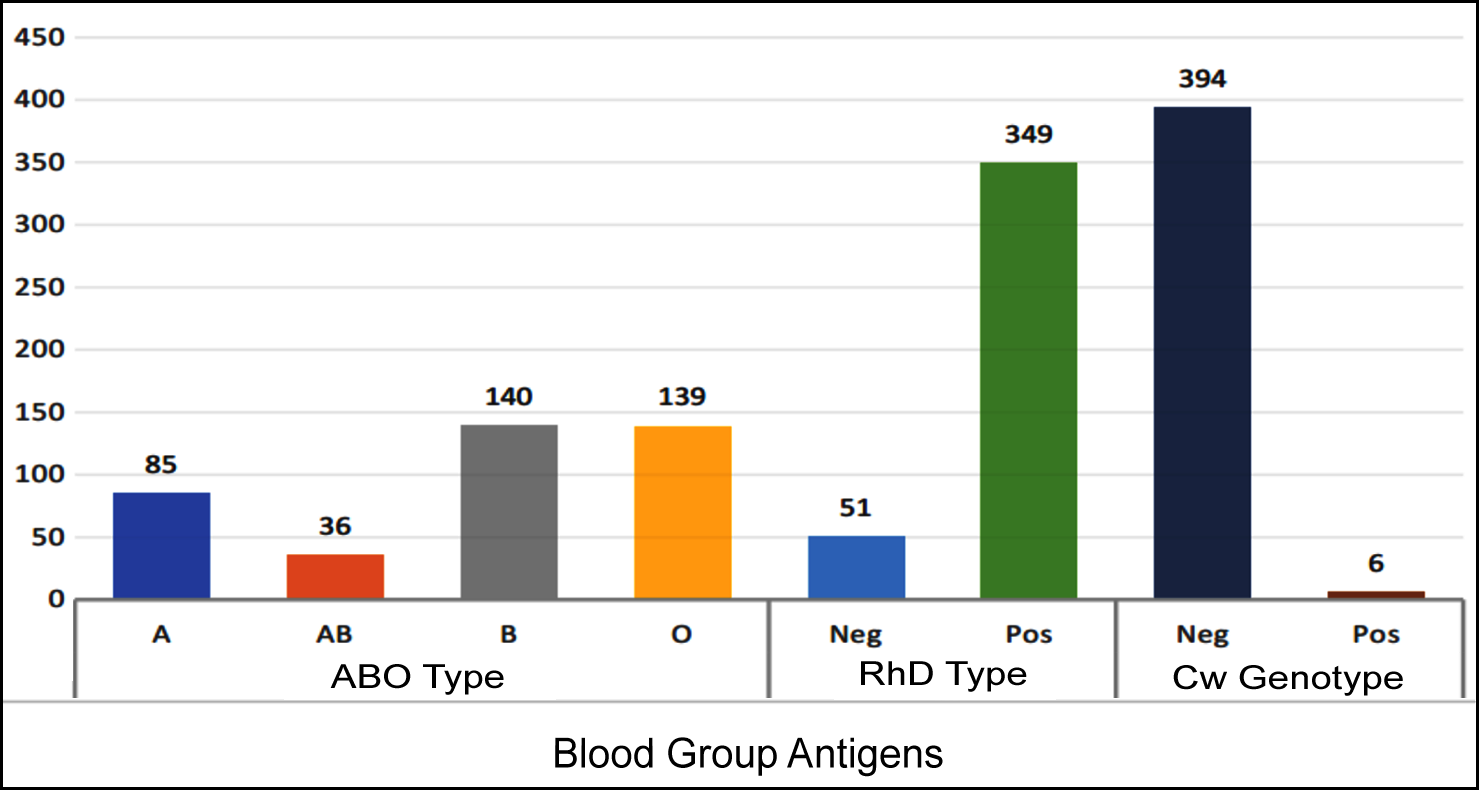 Figure 1: Distribution of blood group antigens in population.
Figure 1: Distribution of blood group antigens in population.
Table I: Demographic features of study participants (n=400).
|
Characteristics |
Frequency (%) |
|
Age |
|
|
18-25 years |
124 (31.0) |
|
26-35 years |
162 (40.5) |
|
36-45 years |
97 (24.2) |
|
46-50 years |
16 (4.0) |
|
51-65 years |
1 (0.3) |
|
Gender |
|
|
Male |
379 (94.8) |
|
Female |
21 (5.2) |
|
Ethnicity |
|
|
Balochi |
1 (0.3) |
|
Gilgiti |
7 (1.8) |
|
Kashmiri |
26 (6.4) |
|
Others |
39 (9.7) |
|
Pashtuns |
43 (10.7) |
|
Punjabi |
271 (67.8) |
The heritability of the Rh Cw antigen adheres to autosomal codominance and demonstrates partial, albeit not absolute, linkage disequilibrium with other Rh antigens. Notably, there is relatively robust linkage disequilibrium between Cw and D, implying that individuals expressing the Cw antigen are more prone to exhibit D positivity and vice versa. Nevertheless, exceptions to this linkage occur due to genetic recombination events. This fact was assessed by applying McNemar test on genotypic expression of Rh D and Rh Cw which showed all those who inherited Rh D also had Rh Cw as evident in Table II.
DISCUSSION
The distribution of blood group antigens on RBC varies significantly among different populations and ethnicities and is of particular importance for the discipline of transfusion medicine, genetic research, and organ transplantation. It is crucial to determine the prevalence of various blood group antigens (including rare antigens) in different populations for safe transfusion practices. Alloimmunisation caused by RBC incompatibility can lead to varied degrees of severe HDFN and haemolytic transfusion responses. All blood banks should have the necessary data on the various RBC antigens in their local population to minimise alloimmunisation in multi-transfused patients, pregnant women, and young females. This study conducted a survey at the molecular level to determine the frequency of Rh Cw antigen in the studied population.12
The knowledge about the distribution of clinically significant rare antigens like Rh Cw antigen is not only crucial for blood banks to carry out routine transfusion services but also in resolution of blood group discrepancies with an ultimate aim of provision of compatible blood to the patients. Worldwide so many studies have been conducted at the molecular level to determine the frequencies of various blood group antigens.13
However, the scarcity of available data in Pakistan regarding the distribution of various blood group antigens is a major setback for local blood banks. Therefore, this study being the pioneer study reporting the frequency of distribution of Rh Cw antigen in the Pakistani population is a starting step for further work in this regard.
Table II: Relationship of blood groups with Rh Cw antigen.|
Blood Group of participants |
Genotypic Expression of Rh Cw |
p-value (Chi-Square test) |
|||
|
ABO blood group |
Rh blood group |
Rh Cw positive n (%) |
Rh Cw negative n (%) |
Total n (%) |
|
|
A |
Rh D positive |
79 (19.75) |
10.25 (2.56) |
80 (20) |
<0.001 |
|
Rh D negative |
5 (1.25) |
0 (0) |
5 (1.25) |
||
|
Total |
84 (21) |
1 (0.25) |
85 (21,25) |
||
|
B |
Rh D positive |
116 (29) |
3 (0.75) |
119 (29.75) |
<0.001 |
|
Rh D negative |
21 (5.25) |
0 (0) |
21 (5.25) |
||
|
Total |
137 (34.25) |
3 (0.75) |
140 (35) |
||
|
O |
Rh D positive |
116 (29) |
1 (0.25) |
117 (29.25) |
<0.001 |
|
Rh D negative |
22 (5.5) |
0 (0) |
22 (5.5) |
||
|
Total |
138 (34.5) |
1 (0.25) |
139 (34.75) |
||
|
AB |
Rh D positive |
32 (8) |
1 (0.25) |
33 (8.25) |
<0.001 |
|
Rh D negative |
3 (0.75) |
0 (0) |
4 (1) |
||
|
Total |
35 (8.75) |
1 (0.25) |
36 (9) |
||
|
p <0.05 is considered to be significant. |
|||||
From the distribution of Rh antigens in different populations in the world it became evident that the frequency of Rh Cw antigen in the Pakistani population (1.5%) is nearly similar to the frequency of Cw found in Greeks (1.48%) and English populations (1.29%). It also closely resembles the frequency of Cw antigen in the North Indian Population (1.25%).
The data derived from multiple studies provide insights into the prevalence of the Cw antigen across diverse ethnicities and populations. Varying prevalence of the Rh Cw antigen across diverse ethnicities, ranging from 0.95% in South Brazil to 9% in Latvians signifies the population-specific nature of this antigen's distribution.
Reid et al. noted a Cw antigen prevalence of 2% in Caucasians and 1% in Black populations, indicating a comparatively low occurrence within these ethnic groups.13 Conversely, Race et al. documented a higher prevalence among Latvians (9%) and Fins (4%), suggesting variability within Caucasian population.14 Simić et al. observed a Cw antigen prevalence of 3.5% among Serbian individuals, akin to Caucasians but slightly lower than Latvians, indicating regional distinctions within Caucasian population.15 Jain et al. found a relatively low prevalence of 1.25% in Northern India,16 lower than some Caucasian population but higher than Black populations. Costa et al. reported a Cw antigen prevalence of 0.95% in South Brazil, lower than in most other population studied.17 This variance underscores the heterogeneity in Cw antigen prevalence among different ethnic groups. While Latvians exhibit a relatively high prevalence, South Brazilians demonstrate a notably lower occurrence.
The observed disparities may stem from genetic factors, population migration, and environmental influences. Further investigation is warranted to elucidate the underlying mechanisms contributing to the variations in Cw antigen prevalence among diverse populations.
Statistics showed that there is a significant correlation between Rh Cw and RhD antigens which supports the Callender and Race findings about the expression of Cw antigen more frequently with CDe/CDe (Rh1Rh1) genotypic group, less frequently with CDe/cde (Rh1rh) and CDe/cDE (Rh1Rh2) groups, and not at all in the cde/cde (rh rh) and cDE/cde (Rh2rh) groups. It showed that Cw antigen is expressed frequently when antigen Rh D is present. A proposed hypothesis that may explain this phenomenon is that it may be due to the close vicinities of their genes on the Chromosome 1 (as RHD and RHCE genes. are organised in tandem on chromosome 1 at p34-p36 and Cw antigen is the result of A > G at 122bp on exon 1 of RHCE gene). This hypothesis is supported by the evidence that the regulation of gene expression can be influenced by neighbouring genes and the expression of certain antigens is determined by the interaction of multiple genes.18
Literature showed that pregnant women may exhibit this rare anti-Cw antibody in the frequency of 0.1% and alloimmunised women have a 2% chance of developing HDN. In view of the clinical significance of anti-Cw antibodies in causing mild to moderate transfusion reactions as well as HDFN, it is suggested that Cw antigen-positive screening cells should be included in the antibody screening and identification panel. If this antibody is identified in a pregnant woman, then the titers should be strictly observed throughout the pregnancy in order to avoid HDFN.18
Mass level study should be conducted at multiple centres including equal participation of both genders of multiple ethnicities. This diverse study population will be used to determine accurate Rh E, C, c, and, e frequencies in various ethnicities of the Pakistani population and its relationship with Rh Cw.
This is the first-ever study for the detection of genotypic frequency of Rh Cw antigen in Pakistan but there was a limited representation of the various communities of the country. The study was conducted on a small scale without equal distribution of genders (limited representations of female donors). Therefore, more studies with larger sample size and fair distribution of the genders are required for further analysis and validation of the results.
CONCLUSION
Only 1.5% of the random blood donors of Northern Pakistan had the Rh Cw antigen Genotype. In this study, blood group B was found to be most prevalent, followed by blood group O. In the studied population, significantly high frequency of Rh Cw was seen in population strata that was Rh D positive.
ETHICAL APPROVAL:
Approval from the institutional ethical review committee was sought prior to the conduct of the study.
PATIENTS’ CONSENT:
Informed consent was taken from patients prior to sampling.
COMPETING INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
SF: Conception, and manuscript writing.
AMA: Substantial contributions to acquisition of data, data analysis, and interpretation of data.
AA, RAKL: Technical support and manuscript review.
NS, RG: Review and final drafting.
All authors approved the final version of the manuscript to be published.
REFERENCES
- Floch A. Molecular genetics of the Rh blood group system: Alleles and antibodies-a narrative review. Ann Blood 2021; 6:29. doi:10.21037/aob-20-84.
- Floch A. Maternal red blood cell alloimmunisation Working Party, literature review. RH blood group system: Rare specificities. Transfus Clin Biol 2021; 28(3):314-20. doi: 10. 1016/j.tracli.2021.04.007.
- Pahuja S, Sehgal S, Sharma G, Chandra J, Parakh N, Singh M, et al. Cw Alloimmunization in Multitransfused Thalassemic Patients of North India: Prevalence and Approach to Transfusion. Glob J Transfus Med. 2022; 7(1):59-64. doi: 10.4103/gjtm.gjtm_89_21.
- Macher S, Wagner T, Rosskopf K, Reiterer F, Csapo B, Schlenke P, Klaritsch P. Severe case of fetal hemolytic disease caused by anti-C(w) requiring serial intrauterine transfusions complicated by pancytopenia and cholestasis. Transfusion 2016; 56(1):80-3. doi: 10.1111/trf.13367.
- Scheffer PG, van der Schoot CE, Page-Christiaens GC, de Haas M. Noninvasive fetal blood group genotyping of rhesus D, c, E and of K in alloimmunised pregnant women: Evaluation of a 7-year clinical experience. BJOG 2011; 118(11):1340-8. doi: 10.1111/j.1471-0528.2011.03028.x.
- Li L, Noumsi GT, Kwok YY, Moulds JM, Scott MD. Inhibition of phagocytic recognition of anti-D opsonized Rh D+ RBC by polymer-mediated immunocamouflage. Am J Hematol 2015; 90(12):1165-70. doi: 10.1002/ajh.24211.
- Zhu Z, Ye L, Li Q, Gao H, Tan Y, Cai W. Red cell immuno-hematology research conducted in China. Transfus Med Rev 2017; 31(2):102-6. doi: 10.1016/j.tmrv.2016.11.004.
- Agarwal N, Thapliyal RM, Chatterjee K. Blood group phenotype frequencies in blood donors from a tertiary care hospital in north India. Blood Res 2013; 48(1):51-4. doi: 10.5045/br.2013.48.1.51.
- Fasano RM. Hemolytic disease of the fetus and newborn in the molecular era. Semin Fetal Neonatal Med 2016; 21(1):28-34. doi: 10.1016/j.siny.2015.10.006.
- Torres-Aguilar H, Sosa-Luis SA, Ríos-Ríos WJ, Romero-Tlalolini MLÁ, Aguilar-Ruiz SR. Silent red blood cell autoantibodies: Are they naturally occurring or an effect of tolerance loss for a subsequent autoimmune process? Autoimmunity 2020; 53(7):367-75. doi: 10.1080/ 08916 934.2020.1799989.
- Gautam A. DNA Isolation by chelex method. In: DNA and RNA isolation techniques for non-experts. Cham: Springer 2022; 9-84. doi:10.1007/978-3-030-94230-4.
- Mangwana S, Gohel D, Simon N. Rare Anti-Cw antibody: Two case reports with review of literature. Glob J Transfus Med 2022; 7(1):99-102. doi:10.4103/gjtm.gjtm_92_21
- Reid ME, Lomas-Francis C, Olsson ML. The blood group antigen factsbook. Academic press 2012. doi:10.1016/ C2011-0-69689-9
- Race RR, Sanger R, Fisher R. Blood groups in man. Oxford: Blackwell Scientific; 1968. https://scholar.google.com/ scholar_lookup?title=Blood+Groups+in+Man&author=RR+Race&author=R+Sanger&publication_year=1975 (accessed May 12, 2023).
- Simić S. Frekvencija CW antigena [Frequency of C W antigen]. Bilt Hematol Transfuz 1975; 3(1-2):73-6.
- Jain A, Elhence P, Tripathi A, Pandey H, Agarwal P. Anti- c(w): In a young female patient. A case report with review of literature and frequency of low incidence c(w) (rh8) antigen in north India. Indian J Hematol Blood Transfus 2014; 30(Suppl 1):440-4. doi: 10.1007/s12288- 014-04 58-1.
- Costa SS, Souza Silva TC, Chiba AK, Cruz BR, Langhi Junior DM, Bordin JO. Molecular study of Cw /Cx antigens and frequency of Rh phenotypes in southeast Brazilian blood donors. J Clin Lab Anal 2018; 32(8):e22570. doi: 10.1002/ jcla.22570.
- Malik S, Moiz B. Clinical significance of maternal anti-Cw antibodies: a review of three cases and literature. J Pak Med Assoc 2012; 62(6):620-1.